-
 EPO 3000iu GENERIC
1 × £69.00
EPO 3000iu GENERIC
1 × £69.00 -
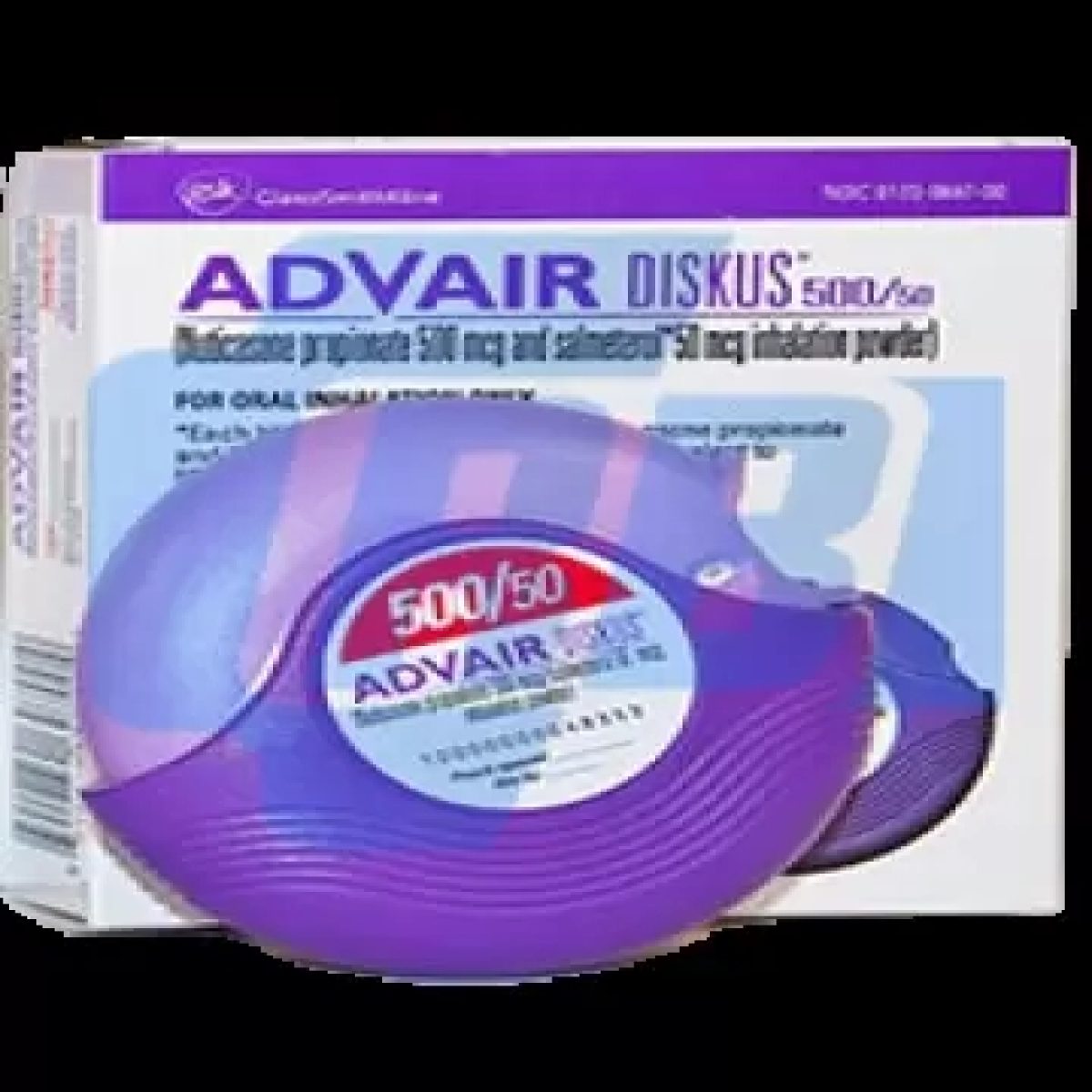
 Seretide Disckus (Advair) 500 mcg Glaxosmithkline
3 × £75.00
Seretide Disckus (Advair) 500 mcg Glaxosmithkline
3 × £75.00 -
 Clenbuterolhydrochlorid Sopharma
1 × £20.00
Clenbuterolhydrochlorid Sopharma
1 × £20.00 -
 FUCIDIN 500 mg Abdi Ibragim
1 × £40.00
FUCIDIN 500 mg Abdi Ibragim
1 × £40.00 -
 BIOSYNERGY RECOVERY3 PEPTIDE BLEND LIFETECH LABS
1 × £259.00
BIOSYNERGY RECOVERY3 PEPTIDE BLEND LIFETECH LABS
1 × £259.00 -
 Clexane 0.4 Sanofi
1 × £44.00
Clexane 0.4 Sanofi
1 × £44.00 -
 Champix 56 tabs Pfizer
2 × £174.00
Champix 56 tabs Pfizer
2 × £174.00 -
 CANTAB PLUS 32/12.5 mg Nobel
2 × £75.00
CANTAB PLUS 32/12.5 mg Nobel
2 × £75.00 -
 Triptorelin 2mg GENERIC
1 × £52.00
Triptorelin 2mg GENERIC
1 × £52.00 -
 Rovamycine 3.000.000 iu Eczacibasi
1 × £42.00
Rovamycine 3.000.000 iu Eczacibasi
1 × £42.00
Subtotal: £1,249.00



Reviews
There are no reviews yet.